 A map of the brain
A map of the brain
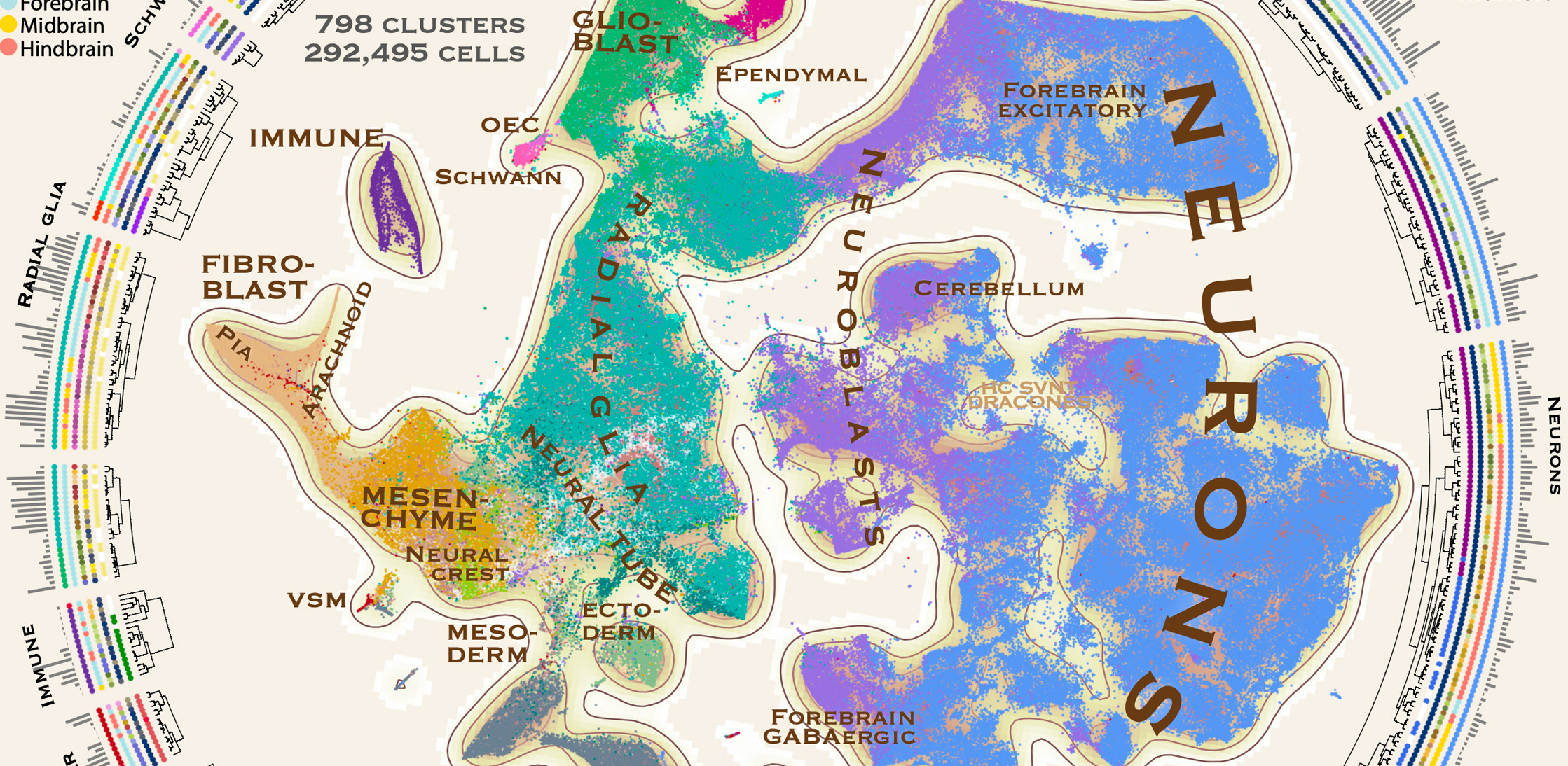
Molecular architecture of the developing mouse brain
Abstract
The mammalian brain develops through a complex interplay of spatial cues generated by diffusible morphogens, cell–cell interactions and intrinsic genetic programs that result in probably more than a thousand distinct cell types. A complete understanding of this process requires a systematic characterization of cell states over the entire spatiotemporal range of brain development. The ability of single-cell RNA sequencing and spatial transcriptomics to reveal the molecular heterogeneity of complex tissues has therefore been particularly powerful in the nervous system. Previous studies have explored development in specific brain regions, the whole adult brain and even entire embryos. Here we report a comprehensive single-cell transcriptomic atlas of the embryonic mouse brain between gastrulation and birth. We identified almost eight hundred cellular states that describe a developmental program for the functional elements of the brain and its enclosing membranes, including the early neuroepithelium, region-specific secondary organizers, and both neurogenic and gliogenic progenitors. We also used in situ mRNA sequencing to map the spatial expression patterns of key developmental genes. Integrating the in situ data with our single-cell clusters revealed the precise spatial organization of neural progenitors during the patterning of the nervous system.